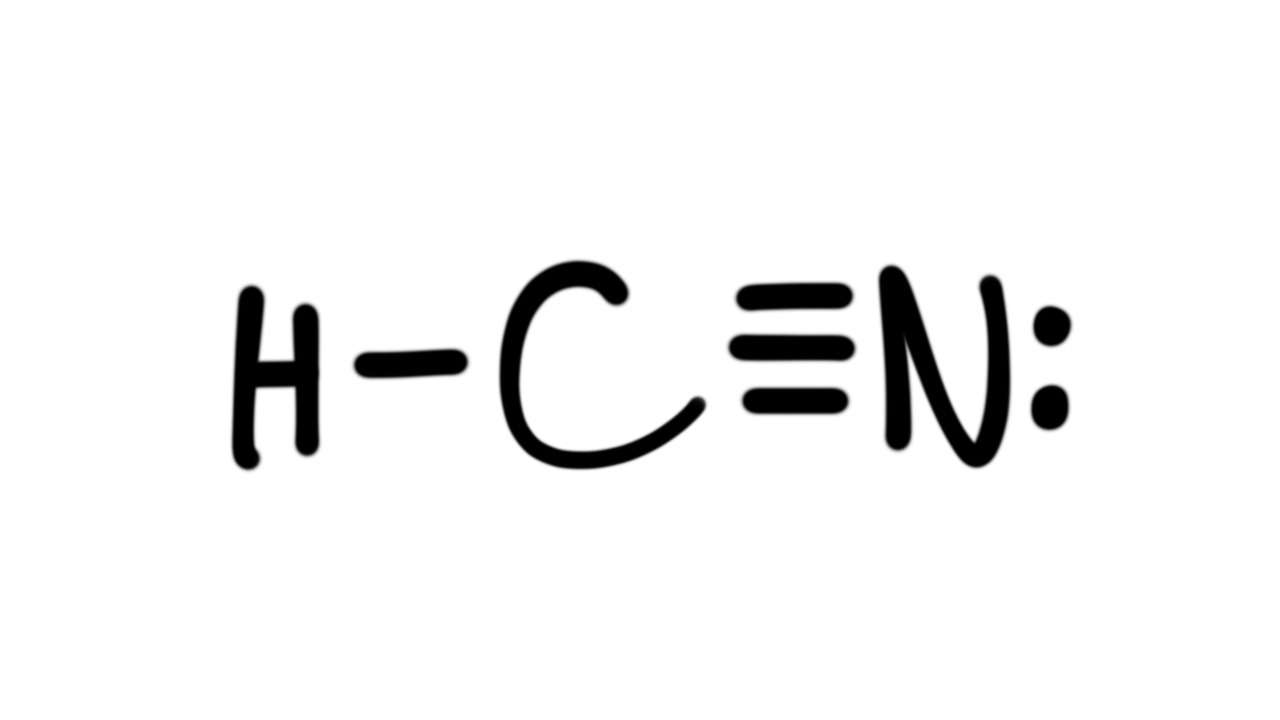
Hydrogen Cyanide YouTube
Chemistry learning made easy.This tutorial will help you deal with the lewis structure and moleculargeometry for hydrogen cyanide (HCN).
Hydrogen Cyanide Molecule Photograph by Laguna Design Pixels
Lewis Structure of Hydrogen cyanide - HCN Chemical data of Hydrogen Cyanide-HCN Physical Properties of Hydrogen cyanide-HCN Chemical Properties of Hydrogen cyanide-HCN Hydrocyanic acid reacts with bases like sodium hydroxide forms sodium cyanide and water. The chemical equation is given below. HCN + NaOH → NaCN + H2O
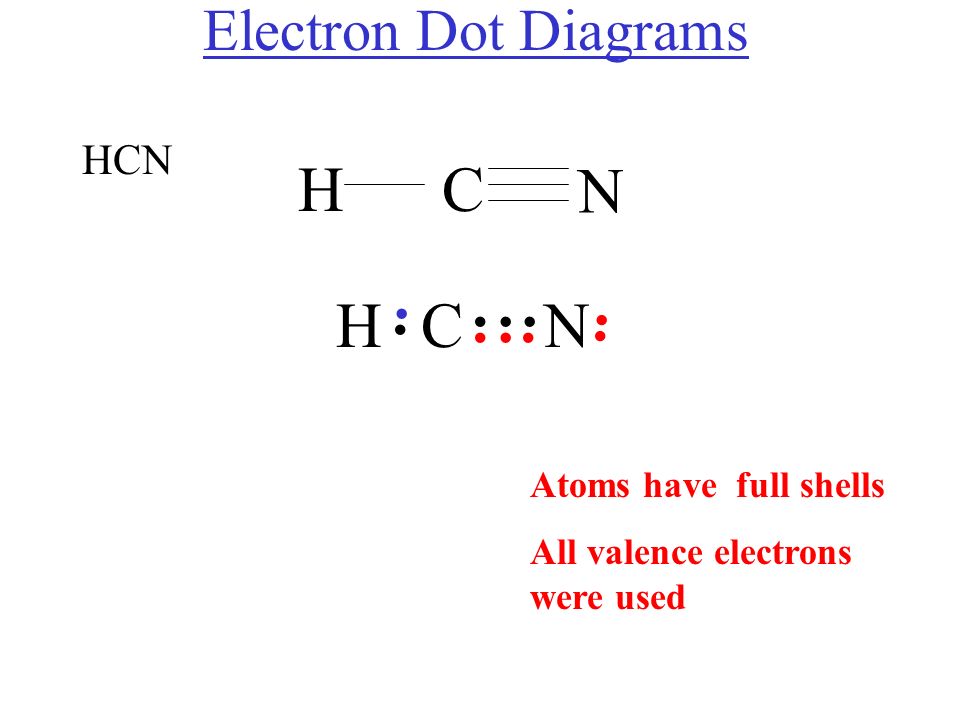
Diagrama De Lewis Hcn Estudiar
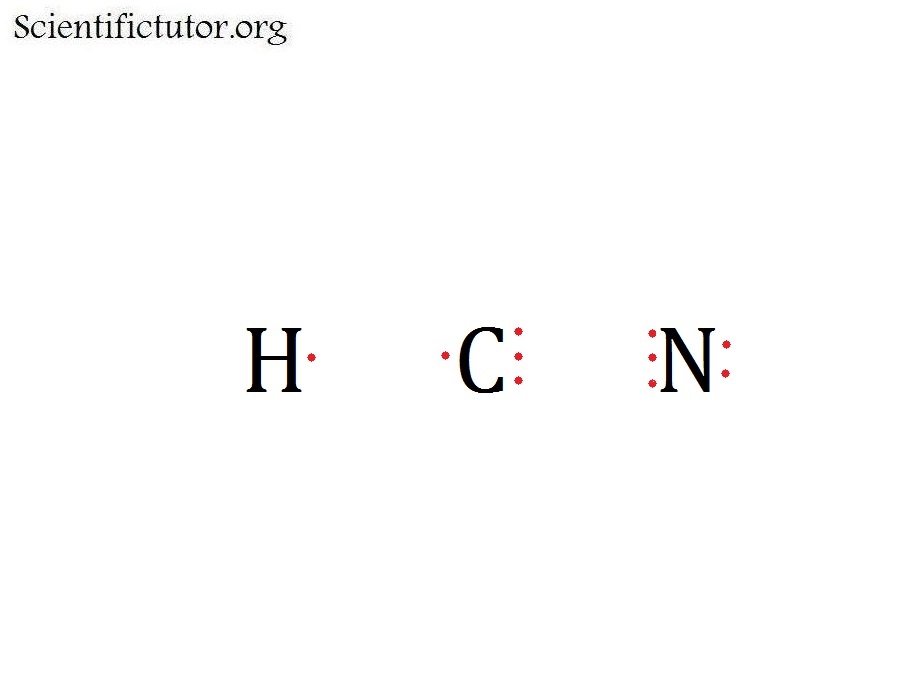
Step 1: The foremost step of creating a Lewis structure is finding the valence electrons. Here we have to find the valence electrons of all three atoms, hydrogen, carbon, and nitrogen.
Hcn Dot Diagram
The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: XeF 6: We place three lone pairs of electrons around each F atom, accounting for 36 electrons. Two electrons remain, and this lone pair is placed on the Xe atom: Exercise 4.2. 2: interhalogens.
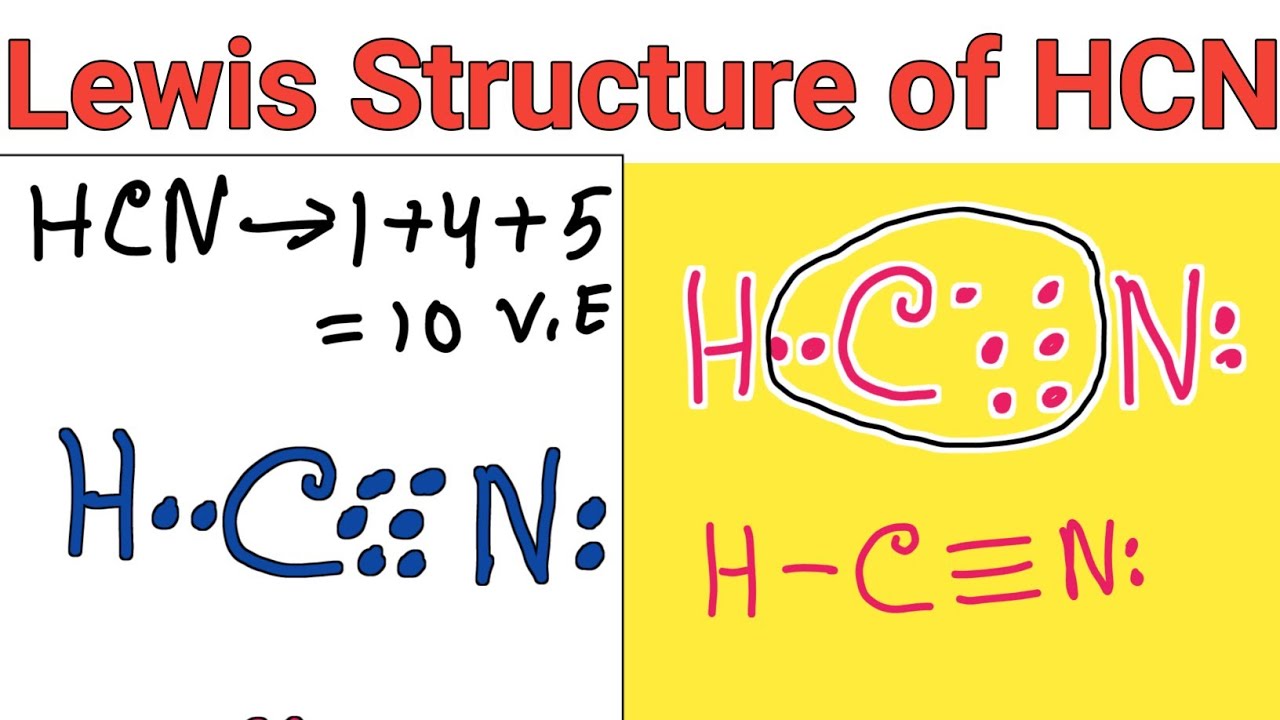
Lewis structure of HCN (Hydrogen cyanide) YouTube
The Lewis structure or dot structure of a hydrogen cyanide (HCN) molecule can easily represent the electron arrangement around the atoms of HCN as it is made by bonding 3 different atoms together. HCN is a linear molecule which is bounded by a hydrogen atom, a carbon atom, and a nitrogen atom.

HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and Polarity Techiescientist
Hydrogen cyanide is a one- carbon compound consisting of a methine group triple bonded to a nitrogen atom It has a role as a human metabolite, an Escherichia coli metabolite and a poison. It is a hydracid and a one- carbon compound. It is a conjugate acid of a cyanide. It is a tautomer of a hydrogen isocyanide. ChEBI

Draw the Lewis dot structure of Hydrogen cyanide (HCN) molecule
The Lewis Structure of HCN. When creating the Lewis structure of HCN, it is important to start by identifying the central atom, which in this case is carbon. Nitrogen and hydrogen atoms are then placed around the carbon atom, with the hydrogen atoms forming a single bond with the carbon atom and the nitrogen atom forming a triple bond with the.

Bond formation, hydrogen cyanide molecule Stock Image C028/6480 Science Photo Library
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

HCN Lewis StructureHydrogen Cyanide (HCN) Lewis Dot StructureDraw Lewis Structure of HCN
There is a single covalent bond between the hydrogen and carbon atom, represented by two dots, :, each of which represents a shared electron; a triple covalent bond between the carbon and nitrogen atom, represented by three pairs of dots, :::, representing three pairs of shared electrons, and a lone pair of electrons on the nitrogen atom, repres.

Chemical Structure Hydrogen Cyanide Hcn Stock Vector (Royalty Free) 2125623392 Shutterstock
A triple bond forms when three electron pairs are shared by a pair of atoms, as in carbon monoxide (CO) and the cyanide ion (CN -): Writing Lewis Structures with the Octet Rule. For very simple molecules and molecular ions, we can write the Lewis structures by merely pairing up the unpaired electrons on the constituent atoms. See these examples:
[Solved] Draw the Lewis structure for it as well. 3. Hydrogen cyanide (HCN)... Course Hero
Hey Guys!In this video, we will look at the Lewis Structure of Hydrogen Cyanide having a chemical formula of HCN. The molecule is made up of one Hydrogen ato.
Hydrogen Cyanide Molecule Photograph by Laguna Design Pixels
Lewis Structure and Hybridization of HCN (hydrocyanic acid, hydrogen cyanide) HCN has a hydrogen atom single-bonded to a carbon atom, and that carbon atom is triple-bonded to a nitrogen atom. These are all non-metals, so the bonds are covalent and HCN is therefore a covalent (aka Molecular) structure.
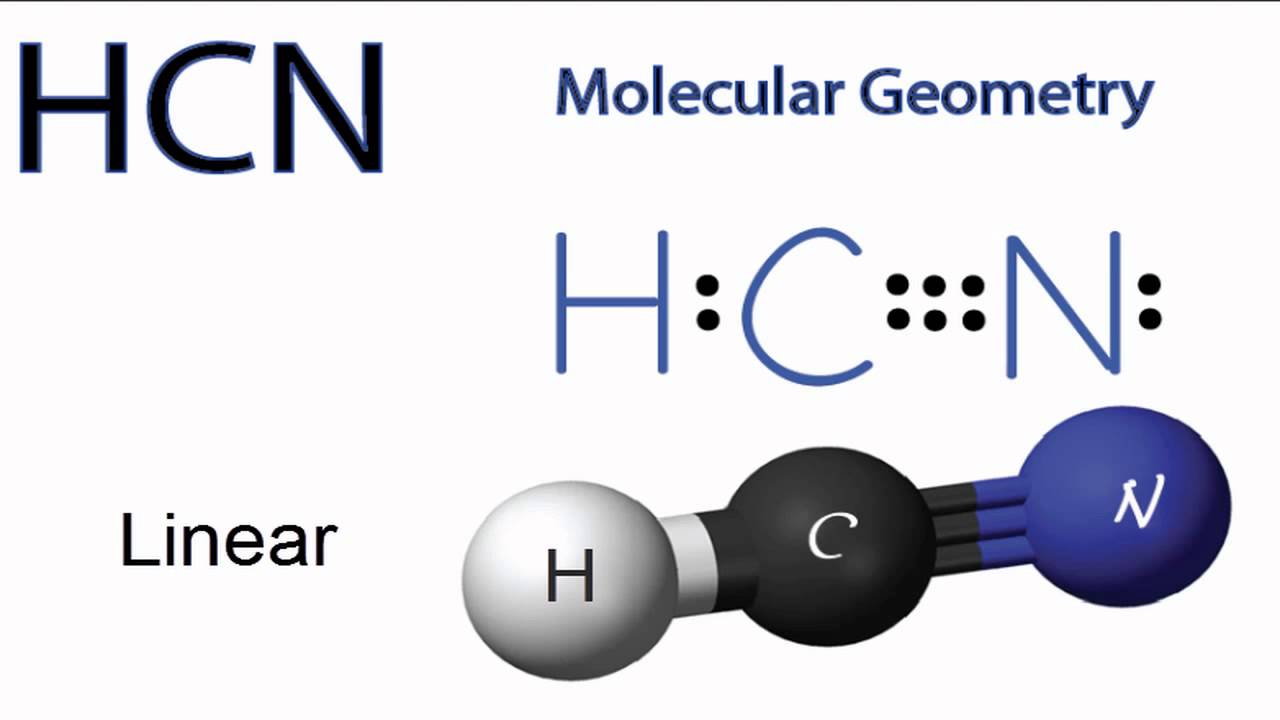
HCN Molecular Geometry YouTube
For the HCN Lewis structure, calculate the total number of valence electrons for the HCN molecule. After determining how many valence electrons there are in HCN, place them around the central.

Hydrogen Cyanide Lewis Structure
5.3C: HCN H C N. Page ID. HCN, hydrogen cyanide, is a volatile and poisnous compound with distinguished bitter odor. It is linear molecule with a triple bond between C and N atom and has bond angle of 180 degrees. It can be found in fruits that have pits due to the fact that they contain small amounts of cyanohydrins which slowly releases.
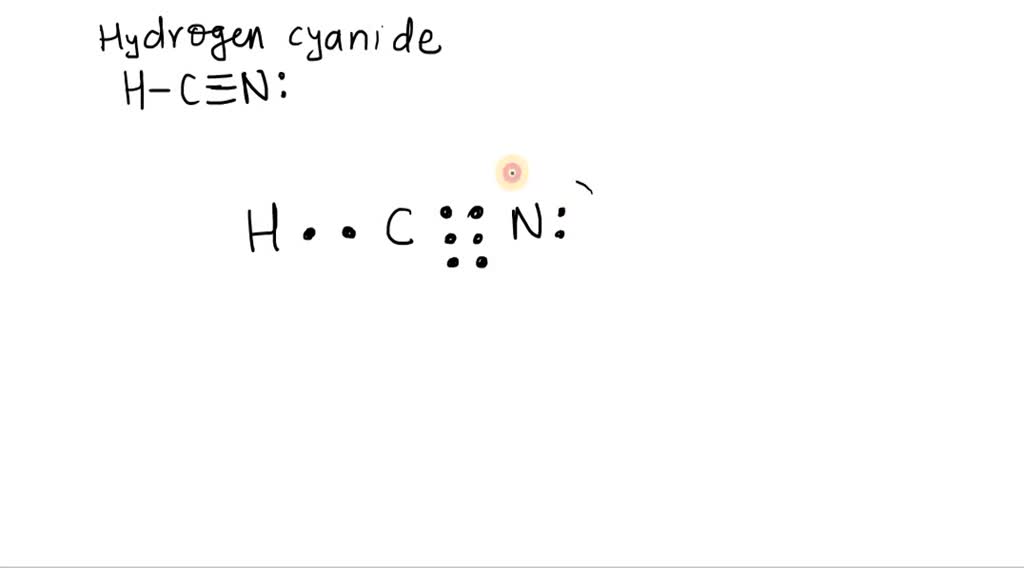
SOLVEDThe Lewis structure for hydrogen cyanide is HC ≡N Draw circles enclosing electrons to
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

HCN Lewis Structure (Hydrogen Cyanide) Molecules, Chemical formula, Lewis
To start with making the Lewis Structure of HCN, we will first determine the central atom. And then place the remaining atoms in the structure. As Carbon is the least electronegative atom in this molecule, it will take the central position. Place the Hydrogen and Nitrogen atoms on both terminal sides of the Carbon like this: