Lewis Dot Structures Worksheet / Lewis Dot Diagram For N Wiring Site
Lewis Dot of Methanol (methyl Alcohol) CH 3 OH. Back: 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons).

Spice of Lyfe Methanol Chemical Equation
Drawing the Lewis Structure for CH 3 OH. For the CH 3 OH Lewis structure there are a total of 20 valence electrons available. Remember that Hydrogen (H) only needs 2 valence electrons for a full outer shell. The OH group is attached to the Lewis structure for CH 3 OH as writen in the chemical formula. Put three hydrogens and the OH around the.

i. What is the Lewis structure for CH3CHCHOH? (If resonance is present
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

Hybridization of CH3OH YouTube
The molecule methanol (methyl alcohol) has the structure CH3OH, and contains fourteen valence electrons (four for carbon, six for oxygen, one each for the four hydrogens). Students should be able to construct a Lewis dot structure, making sure that the carbon and oxygen atoms have enough electrons to satisfy the octet rule (eight surrounding.

Solved Draw the product formed when the structure shown
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

CH3O Lewis Structure How to Draw the Lewis Structure for CH3O YouTube
A step-by-step explanation of how to draw the CH3OH Lewis Structure.When you see a carbon with an OH attached (like CH3OH, C2H5OH, etc.) that means that he O.

How to Draw the Lewis Structure for CH3OH (Methanol) YouTube
CH3OH Lewis structure October 31, 2023 by Deep The information on this page is fact-checked. CH 3 OH Lewis structure CH 3 OH (methanol) has one carbon atom, four hydrogen atoms, and one oxygen atom. In the CH 3 OH Lewis structure, there are three C — H bonds, one C — O bond, and one O — H bond. And on the oxygen atom, there are two lone pairs.

CH3OCH3 Lewis Structure How to Draw the Lewis Structure for CH3OCH3
May 22, 2023 by Jay Rana Ready to learn how to draw the lewis structure of CH3OH? Awesome! Here, I have explained 6 simple steps to draw the lewis dot structure of CH3OH (along with images). So, if you are ready to go with these 6 simple steps, then let's dive right into it!
Draw The Lewis Structure Of Ch Oh Vanspigsuedeauthentic My XXX Hot Girl
Watch on Steps of drawing CH3OH lewis structure Step 1: Find the total valence electrons in CH3OH molecule In order to find the total valence electrons in CH3OH molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as oxygen atom.
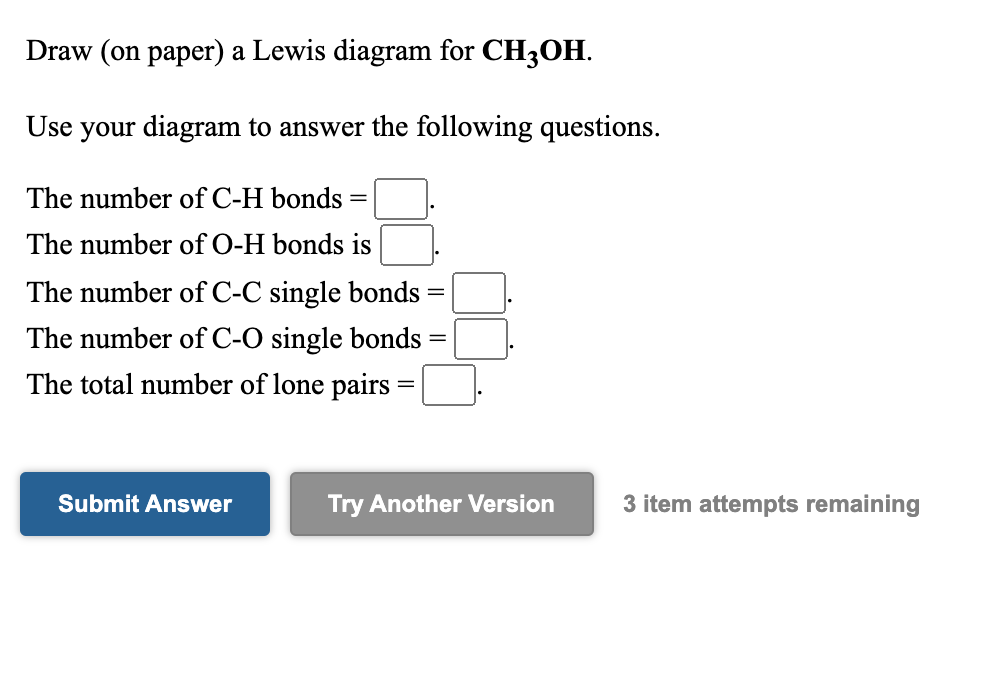
Solved Draw (on paper) a Lewis diagram for CH3OH. Use your
A step-by-step explanation of how to draw the CH3O- Lewis Dot Structure.Note that you should put the CH3O- Lewis structure in brackets with as 1- on the outs.
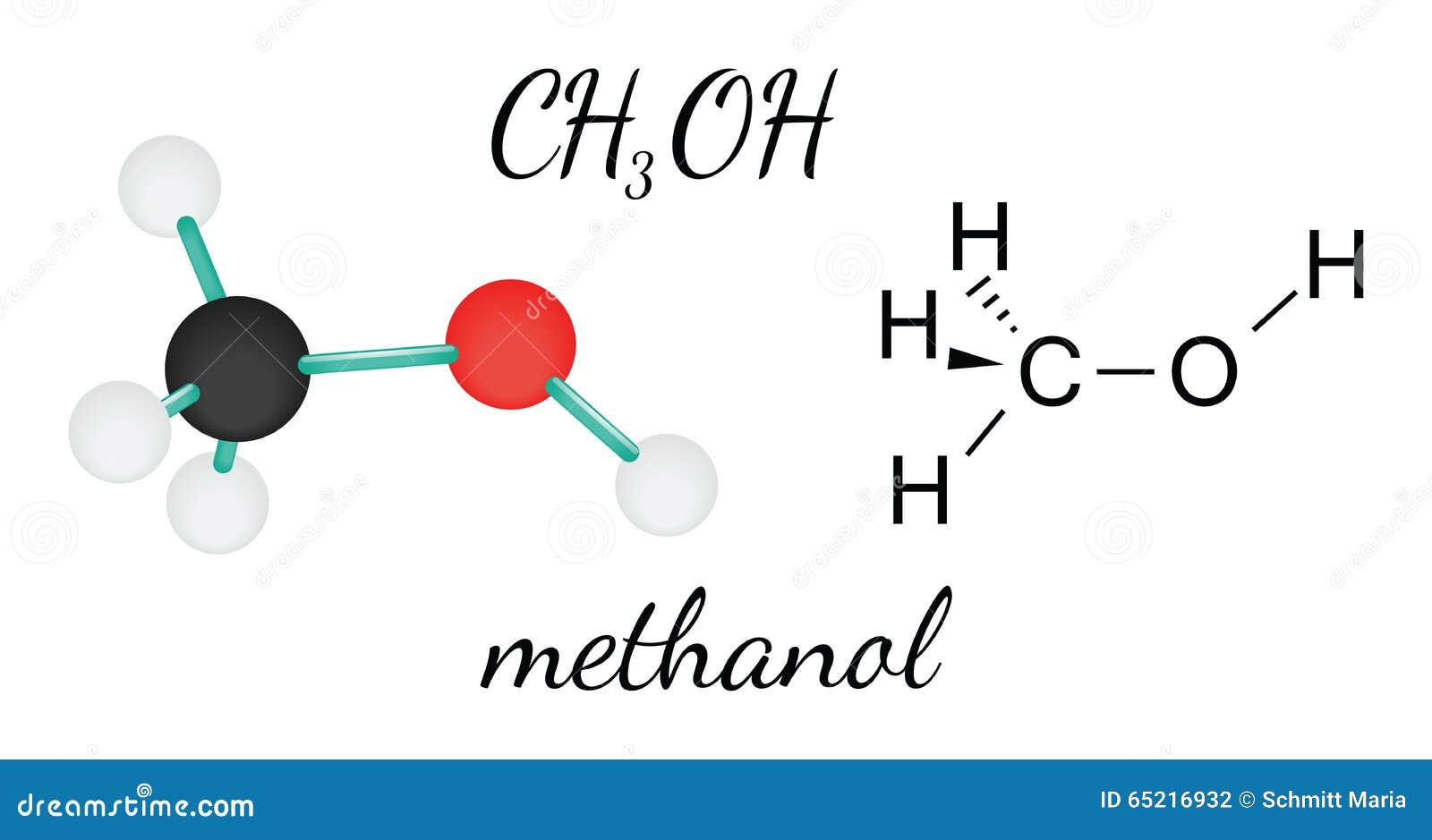
CH3OH methanol molecule stock vector. Illustration of hydrogen 65216932
An isolated carbon owns 4 valence electrons. The bound carbon in methanol owns (½ x 8) = 4 valence electrons: formal charge on carbon =. (4 valence electron on isolated atom) - (0 nonbonding electrons) - (½ x 8 bonding electrons) = 4 - 0 - 4 = 0. So the formal charge on carbon is zero. For each of the hydrogens in methanol, we also get a.
CH3OH Lewis Structure How to Draw the Lewis Structure for CH3OH
Lewis Dot Structure of CH3OH (Methanol) kentchemistry.com 25.1K subscribers Subscribe Subscribed 127K views 12 years ago Every Video I quickly take you through how to draw the Lewis.
Lewis structure, Hybridization, and Molecular Geometry of CH3OH by
The Lewis structure of CH3OH, also known as methanol, is a representation of the molecule's bonding and electron distribution. It provides valuable insights into the molecule's geometry, hybridization, and polarity. Let's explore the step-by-step process of determining the Lewis structure of CH3OH. Calculation of Valence Electrons
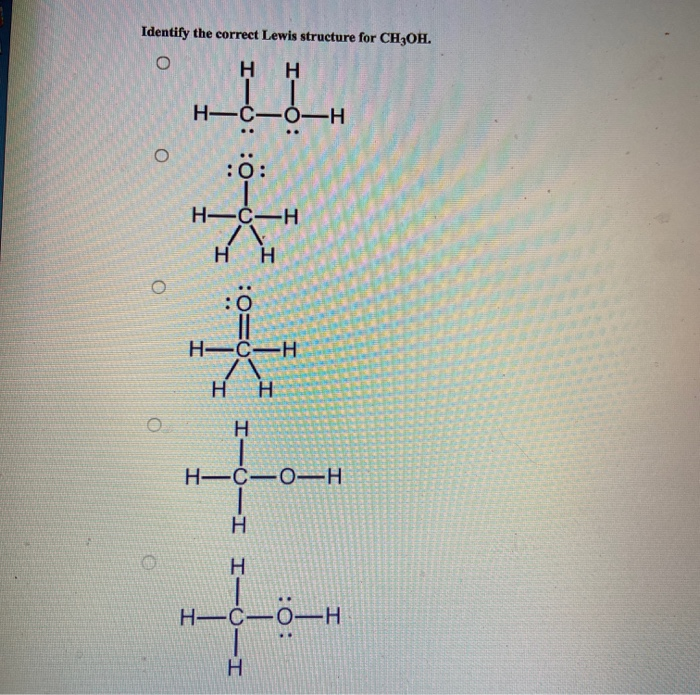
Solved Identify the correct Lewis structure for CH3OH. o H H
Lewis dot structure is a pictorial representation of the molecule, it's bonding with other atoms and the arrangement of atoms in the compound. It helps in knowing the number of bonded electrons, lone pairs, and the compound's molecular shape.

Lewis dot structure of CH3OH Quizlet
1.93K subscribers Subscribe 2K views 1 year ago Lewis Structure Hey Guys! For today's video, we are going to study the Lewis dot structure of Methanol. It has a c Lewis Structure of SO4.

Estructura De Lewis Ch3oh Compuesto
What is the Lewis structure of [//substance:CH3OH//]? Natural Language Math Input Extended Keyboard Examples Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports, finance, music…